Linguistic Validation
We adapt and validate COA instruments to confirm that concepts, instructions, and response options are interpreted correctly and function as intended across languages and cultural contexts.
Have questions?
Get the answers you’re looking for. Contact us. No obligation.
Get In Touch
Phone: +1.833.726.8486 Helpdesk: info@santium.com
Supporting Solutions
COA Translation and Adaptation for Global Studies
LINGUISTIC VALIDATION
Linguistic validation is a structured process used to adapt Clinical Outcome Assessments (COAs) for use in multilingual studies. It confirms that patients, caregivers, and clinicians interpret concepts, instructions, and response options as intended, without altering the measurement goals of the instrument.
We specialize in clinimetrics, hence our process reaches beyond translation. Each step—concept-focused translation, clinician review, reconciliation, cognitive debriefing, and harmonization—is designed to maintain conceptual fidelity and functional usability across languages. This in turn supports more reliable data collection in global clinical research.
We work with the World’s largest publishers and authors, translating complex neuropsychological assessment batteries and simple rating scales into more than 140 languages.

Translation & Reconciliation
Concept-focused forward and back translations of COA instruments, followed by expert-led reconciliation to resolve linguistic discrepancies, align conceptual meaning, and prepare each item for cognitive testing. This step supports measurement integrity before pilot testing.
Clinician Review & Harmonization
Subject-matter specialists review medically sensitive content, rating instructions, anchors, and test items to assess clinical appropriateness. Cross-language harmonization aligns terminology and item intent across dialects, supporting consistent examinee comprehension and score interpretation.

Cognitive Debriefing
Semi-structured interviews with native speakers from the target population to evaluate comprehension, cultural relevance, item content usability and performance. Feedback is analyzed to identify misunderstood concepts, ambiguous wording, and potential barriers to accurate self-reporting and observation.
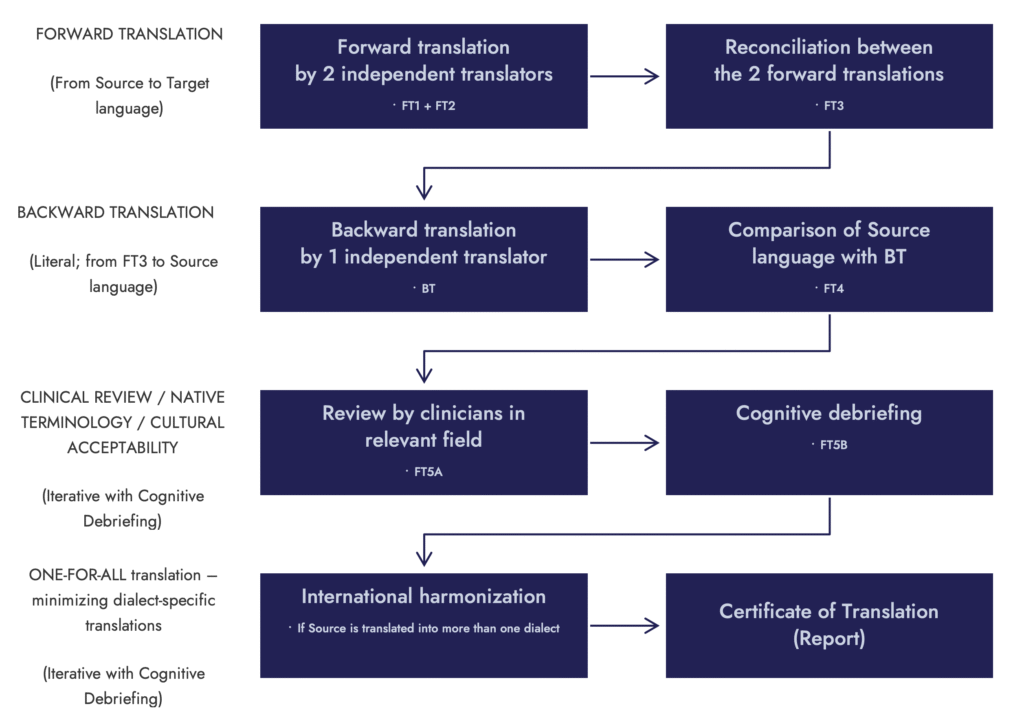
Linguistic Validation Process
Linguistic validation follows a structured, stepwise methodology designed to evaluate how COA items function in new languages. Each stage focuses on a different aspect of conceptual accuracy, cultural relevance, and usability, starting with translation, and extending through patient testing and item-level adaptation where needed.
The process includes concept-focused translation with reconciliation, cognitive debriefing interviews to test real-world comprehension, and trans-creation of items that require deeper cultural or linguistic adjustment. These steps work together to keep COA instruments performing as intended when deployed in multilingual research studies and in international clinical settings.
Linguistic Validation Methodology
The standard methodology to translate Clinical Outcome Assessments (COAs):
Cognitive Debriefing
Cognitive debriefing evaluates how well patients, caregivers, and clinicians understand COA items when applied in real-world contexts. While the standard approach focuses on basic comprehension checks, Santium conducts a deeper analysis of item performance—examining conceptual interpretation, task demands, cultural influences, and potential sources of measurement error.
Our interview methods go beyond confirming whether respondents “understand the words.” We explore how concepts are interpreted, how response options are chosen, and whether any linguistic or cultural factors could alter symptom reporting, observed behaviors, task execution and scoring. This allows us to identify issues that traditional debriefings often miss and deliver revisions that improve the functional use of the instrument in multilingual studies.
Participant selection
We recruit native-speaking participants who match, or closely relate to, the target population’s characteristics—age, condition, education level, and relevant cultural factors—and evaluate how the instrument performs in the intended context of use.
Interview Methods
In cognitive debriefing, we interview native speakers who reflect the target population’s age, condition, and cultural characteristics. Using a combination of think-aloud methods and structured probing, we assess how participants interpret item concepts, instructions, and response scales.
Feedback is analyzed to identify misunderstandings, ambiguous concepts, task-related challenges, and cultural influences that may affect reporting accuracy. Findings are consolidated using predefined decision rules to determine which refinements improve conceptual clarity without altering measurement intent.
The resulting insights directly inform item-level trans-creation and guide the final wording used in multilingual clinical studies.
Documentation
We prepare standard project reports and customized documentation and materials based on customer’s requirements. Some examples include:
- Standard project report
- Interview transcripts or structured notes
- CD summary report with participant feedback
- Recommended wording refinements
- Rationale for each change
- Final revised item set for harmonization
When is Cognitive debriefing necessary?
If you need a linguistically validated translation of a clinical outcome assessment, then cognitive debriefing is necessary. Linguistic validation means dual front and back translation, combined with cognitive debriefing. Cognitive debriefing is the “validation” part of the Linguistic Validation process. Without cognitive debriefing, the translation is not validated. For anything other than COAs, whether cognitive debriefing is needed depends on the purpose of the test, or survey, the complexity of the concepts, and the risk of misinterpretation.
In the Life Sciences sector, cognitive debriefing is especially important whenever patient understanding influences clinical decisions, trial data integrity, or cross-cultural comparability. Santium uses cognitive debriefing to identify comprehension issues, cultural mismatches, and interpretation differences that standard translation cannot detect.
Cognitive debriefing is commonly needed when:
- Patients must report about symptoms, behaviors, or quality of life (e.g., PROs).
- Consistent patient understanding is required across languages for study outcomes.
- Concepts, examples, or cultural references may not transfer directly.
- Participant interpretation affects data quality or endpoint reliability.
- Instruments are used in new cultural or demographic groups.
When is Cognitive debriefing Not necessary?
Cognitive debriefing is generally not required when:
- The material is not a COA (e.g. PRO, ClinRO, ObsRO or PerfO in any format).
- The content is only for clinicians and staff (e.g., user manuals, training materials, etc.).
- The tool does not inform clinical, safety, or other high-impact decisions.
- The translation is for information or administration only.
- There is no cross-cultural or multilingual use.
Transcreation & Cultural Adaptation
In the context of translation and localization, transcreation represents the point at which linguistic equivalence alone is insufficient, and culturally grounded adaptation is required to preserve the item’s intent and usability for the target population. In other words, item transcreation is used when a direct translation cannot capture the original concept or when wording is not culturally or linguistically appropriate for the target population. This includes cases where an item’s meaning, examples, or task expectations do not align with local language use, everyday experience, educational curricula, age-related knowledge, or culturally relevant behaviors.
Our approach prioritizes preserving the instrument’s conceptual intent and psychometric purpose while adapting wording so that respondents interpret the item, task demands, and response options as intended. All adaptations are carefully justified, guided by subject-matter experts and/or instrument developers, documented, and reviewed to maintain consistency with the instrument’s conceptual framework.
All adaptations are documented with rationale, conceptual notes, and reviewer comments for audit readiness.
Concept definitions and construct alignment
- Adaptations follow documented concept definitions and item intents.
- We check for shifts in cognitive burden or interpretation that may affect validity.
- Localized translation is preferred whenever it preserves meaning and function.
Close collaboration with developers and subject-matter experts
When needed, adaptations are reviewed with clinical SMEs and/or COA developers to confirm alignment with the intended construct.
Factors that often initiate transcreation
- Idiomatic expressions or figurative language.
- Culturally unfamiliar activities or examples.
- Educational level differences that affect item comprehension.
- Vocabulary or grammar structures that don't map 1:1 across languages.
- Items describing behaviors not typical in the target population.
transcreation is not appropriate when...
- A shift in the measured construct is unavoidable.
- Core psychometric properties, scoring rules, and item structure is relevant and working.
- Classic localized translation preserves meaning and scoring function.
Linguistic Validation
Finishing the unfinished...
Already have translations but aren’t sure if they’re complete, modified, accurate, certified or fully validated? We can assess what you have, identify gaps, and build on your existing materials instead of starting over. Whether you need missing items translated, outdated versions aligned, or earlier work brought up to LV standards, we’ll help you finish the job.

Cognitive Debriefing
Cognitive debriefing interviews test how real patients, caregivers, or clinicians understand translated COA items. Uncover problematic wording, cultural mismatches, and interpretation issues that affect usability. It is the second half of the Linguistic Validation method; without it, a translation is not linguistically validated.

Clinical Review & Editing
Native clinical specialists review medically sensitive concepts, rating instructions, anchors, and scoring guidance to assess clinical relevance and clarity. Editing focuses on refining terminology, resolving ambiguities, and aligning content with the intended clinical interpretation of each COA item and measurement concept.

Linguistic Review & Editing
Linguistic reviewers refine translated content by correcting terminology, resolving unclear phrasing, and improving structure while preserving the original meaning. Editing focuses on clarity, flow, and accuracy so materials read naturally and meet professional communication standards across scientific, medical, and technical domains.
Proofreading
We perform final quality checks to correct surface-level issues such as grammar, spelling, punctuation, formatting, and typographical errors. Proofreading ensures polished, error-free materials that are ready for publication, distribution, or regulatory submission, complementing earlier editing stages and maintaining consistency across related deliverables.
Glossary & Terminology
We build and maintain specialized terminology databases to unify language across subject-matter, studies and product lines. Controlled vocabularies reduce ambiguity, speed up editing time and strengthen quality assurance throughout the translation lifecycle.

Desktop Publishing
We maintain formatting integrity and brand recognition with polished professional layouts created by native-speaking designers and proofread by an independent reviewer. Translations are clear, accurate, with consistent formatting across manuals, forms, charts, and multi-page technical or scientific documents.







Dedicated Translation Teams
Small project-specific multi-disciplinary groups.

Translation Experience
Training and experience in COA translation.
Preserving psychometric integrity
Expert medical and scientific translators
Our subject-matter expert linguists specialize in medical and scientific translation and terminology. Assisted by the latest translation technologies, we preserve the psychometric integrity of each outcome measure and deliver clear and functional translations that support patient safety and regulatory compliance.
- 3,000+ translators and editors
- 140+ languages supported
- ISO-aligned, audit-ready workflows
- Follow-the-Sun project support across 14 countries
- Specialized dedicated project teams
strengthen your coa Linguistic Validation project
related services
Translatability Assessment
Pre-translation review that identifies linguistic and cultural conflicts before localization begins. This process reduces revision cycles by clarifying and defining ambiguous concepts, resolving inconsistencies in terminology, and identifying content that is not translatable and requires alternate wording, deeper adaptation or transcreation.
Clinimetric Review
Specialized expert review of outcome measures to support adaptation across languages, cultural contexts, and administration modes. To minimize noise in study data, we assess conceptual fidelity, scoring implications, and cross-cultural alignment to keep translated measurement items psychometrically sound and contextually relevant.

Translation & Localization
Clear, accurate, and domain-specific translation for clinical, scientific, technical, and corporate materials. All translations are produced by qualified subject-matter experts and reviewed for terminology accuracy, style consistency, and audience appropriateness, ensuring clarity and usability across languages and regions.
Data Collection & Validation
Cross-language validation of clinical outcome measures for new target populations, usability systems, and instructional content that supports relevant functional use, and target-audience comprehension. It also supports regulatory expectations and a consistent user experience across countries, languages and cultural contexts.
Our approach
The Santium method – where precision meets purpose
Santium’s methodology bridges linguistic accuracy with scientific discipline. Every project follows a validated, ISO-aligned process that preserves meaning, measurement, and usability across languages and contexts.
From concept review to pilot testing, each stage is designed to deliver traceable, reproducible outcomes in more than 140 languages worldwide.
Clients choose Santium for our ability to balance precision with understanding.
We adapt complex materials into content that communicates clearly and performs reliably, whether for regulators, clinicians, or patients, helping organizations build trust and consistency in their markets.
Multimedia and Digital Content Localization
For Training and Onboarding
Multimedia support includes subtitles, captions, and translated audio for rater training modules, e-learning programs, and presentations. Content such as SOP walk-throughs, protocol overviews, and procedural demonstrations is localized for clarity and usability across languages, helping global study teams deploy localized materials.

e-Learning Translation
High-quality translations for training content, rater modules, onboarding courses, and examination materials. Lessons, assessments, and interactive elements are localized for clarity, usability, and cultural relevance, helping global study teams deliver consistent learning experiences across sites, languages, and user groups.

Video Subtitling & Closed Captions
Precise, well-timed subtitles and closed captioning support multilingual training content, including onboarding videos, e-learning modules, and SOP walk-throughs. Terminology and procedural language are translated clearly so multilingual teams can follow demonstrations with ease.

Voiceover Translation (Dubbing)
Transcription, subtitling, voiceover, script localization, video production, and UX review for multimedia used in multi-language training, recruitment and onboarding, education, and research.

Transcription
Accurate, polished and secure transcription for clinical, scientific, and technical content. Audio and video files are converted into clean, structured text that is ready for analysis, review, and multilingual adaptation. Ideal for e-Learning content, training materials, interviews, high-impact meetings, presentations and documentation needing archiving or long-term knowledge retention.

Software Localization
Software localization for eCOA platforms supports your digital assessments, screens, and patient workflows feel native in every language. We adapt UI labels, messages, notifications, and task logic so they stay consistent and aligned with your requirements. Our team understands character limits, branching, scoring dependencies, and audit needs in eCOA systems, delivering a seamless experience across devices.
Video Production
Video production for COA training materials, patient instructions, and site-readiness programs. We create clear, culturally relevant videos that simplify complex concepts, maintain protocol compliance, and support consistent administration across sites and languages. Ideal for onboarding raters, guiding patients and caregivers, and enhancing the usability of COA and eCOA workflows.
linguistic validation
frequently asked questions
Some of the most common questions about linguistic validation, regulatory expectations, data collection and validation of clinical outcome measures.
Translation and Linguistic Validation are two related methodologies but have distinctly different scopes, processes, outcomes and uses.
Linguistic Validation consists of Translation and Cognitive Debriefing. Cognitive Debriefing is the “validation” part of the Linguistic Validation process. If Cognitive Debriefing doesn’t take place, the translation is not linguistically validated; it’s just a translation.
Scope:
Translation focuses on accurately conveying content from one language to another, while Linguistic Validation includes translation but also addresses cultural relevance, understanding, and equivalence across languages.
Process:
Translation typically involves one or two translators and possibly a reviewer. Linguistic Validation involves multiple steps, including translation, back-translation, linguistic and expert review, and cognitive debriefing with target responders.
Outcome:
The outcome of translation is a text that accurately reflects the source material in another language. The outcome of linguistic validation is a culturally and linguistically adapted, comparable/equivalent text–though the wording or parts of the content may differ from source–that is understood in the same way by its target language groups.
Use:
Translation is used for a wide range of documents, including manuals, training content, information booklets, and textbooks. Linguistic Validation is mostly used in the Life Sciences industry, where it’s crucial that instruments like clinical outcomes measures work consistently the same way across different languages and cultures, despite their unique syntax and grammatical structures, and behavioural norms. Linguistic Validation is also used in Academia, e-Learning and Aerospace industries, and in global Marketing and Advertising brand campaigns.
Artificial Intelligence (AI) can assist parts of the linguistic validation process, but it cannot replace the clinical, cultural, or psychometric expertise required to make defensible adaptations. At Santium, AI is used selectively and only in ways that support—not substitute—expert judgment.
WHERE AI IS NOT USED
We do not use AI or MT engines for forward translation of any content that is sensitive in nature, item adaptation, or stylistic editing. These steps require clinical reasoning, cultural sensitivity, and construct-level decision-making that AI cannot (yet) reliably perform. We continue to test AI outputs to evaluate how its ability is evolving. We do use AI for translation of content that only internal exposure or low risk of content misinterpretation.
RESPONSIBLE USE OF AI
AI tools may be used for:
- Generating rough backward translations for early-phase quality checks
- Comparison analyses to quickly flag mistranslations, shifts in meaning, etc.
- Pattern-based review checks (e.g., terminology drift, inconsistent phrasing)
In all cases, AI output is treated strictly as a diagnostic aid, never as a replacement for human translation or validation.
Linguistic validation helps verify that translated clinical instruments function equivalently across languages and cultures. AI cannot evaluate cultural relevance, respondent burden, construct drift, or measurement implications. Human oversight remains essential to safeguard conceptual integrity and regulatory defensibility.
None.
The 2009 Patient-Reported Outcome (PRO) Guidance for Industry, issued by the U.S. Food and Drug Administration (FDA) only briefly touches on the importance of translation and cultural adaptation when patient-reported outcomes measures are used in international clinical trials.
The guidance is primarily focused on ensuring the reliability and validity of PRO measures. Although the guidance is specific to PROs, its content can be readily applied to any type of a clinical outcomes measure (e.g., ClinROs).
Not including a translation methodology in the PRO guidance was intentional, for two key reasons:
1. FDA chose not to limit researchers to a single prescribed method of translation. This is fact: we were part of the working group!
2. The industry was in the process of testing a linguistic validation method developed by a working group representing the International Society for Pharmacoeconomic and Research Outcomes (ISPOR), published it in 2005 (Wild et al., 2005), and at the time still in its infancy stage of adoption. This ISPOR-developed method eventually became the gold standard that is now known and accepted throughout the industry. This is fact: we were also part of this working group!
FDA’s decision not to prescribe a translation method has had a dual effect across the Life Sciences industry:
1. Cognitive debriefing is often omitted from “linguistic validation” projects, mainly due to longer project timelines that are necessary to complete the pilot test. Therefore, a very high percentage of translations used in clinical trials are not linguistically validated.
2. Technological advances in the translation industry made certain steps in the methodology, including its variations, redundant and unnecessary.
After more than 15 years of selective application, the linguistic validation method is due for a practical revision that must include processes for adapting untranslatable content.
Not true. This is sometimes only an unfortunate internal policy. Let’s review what translation equivalency actually means.
Translation equivalency refers to the degree to which a translated content accurately reflects the meaning, intent, and impact of the original source text while being culturally appropriate and understandable in the target language.
Given the prescribed meaning and purpose of “translation equivalency”, the wording of items in an outcomes measure, or a survey, may have to change to accurately convey a particular concept to the target responder, especially when the chosen sample of the measured concept is not specifically relevant to the target responder, or to the context of the responder’s culture or environment.
In some cases, the concept may not exist in the target country altogether, or may only be applicable to other types of responders.
Therefore, when translating clinical outcomes measures, translators and study project managers must unlearn this misconstrued resistance to adapting psychometric instruments.
It will be crucial to validate the translation, at least via thorough cognitive debriefing, before using it in its intended context.
If a user’s manual or any form of instruction accompanies the instrument, or survey, ensure that changes made in the questionnaire(s) are reflected in the administration instructions, including screenshots of sample scoring forms, demo items, etc..
That depends on the nature of the change. Validity can be preserved after adaptation, if changes to source text are done by someone qualified to make that kind of a change.
For example, translators are not qualified to make such changes to COAs. They can, and do, make recommendations, particularly during the translatability assessment stage, which is designed to shed light on content that will need adaptation.
However, the onus to provide clear and accurate instructions on what to change and how to do it, including any necessary training for the translator(s), lies with the party assigning the job to the translator. It is never the translator’s job to “figure it out”. Translators don’t have the appropriate training for modifying source text in clinical outcome measures, regardless how experienced they may be in translation or transcreation.
The validity of a rating scale refers to how well the scale measures what it is intended to measure. Therefore, from a psychometric standpoint, replacing and rewording items is an essential part of preserving the translated instrument’s validity.
A translated clinical outcome measure, even when flawlessly translated to mirror the original source, can instantly lose its validity when some or more of its items are not relevant to the target language speakers and their culture.
For example, items that measure stigmatized concepts, verbal fluency, adaptive behaviours, language development milestones, house chores, or country-specific knowledge across tiered age groups, just to name a few.
Whenever changing source text becomes necessary, it’s critically important to revalidate the scale to ensure that it still measures the intended construct validly and reliably.
More often than not, it’s the author’s representative or agent, or internal policies governing the representative or agent, that doesn’t allow for the changes to be made; not the author.
If the author psychometrically validated the instrument, the author will understand the need for the change, assuming that you have a valid argument to support the change. Also, the author must know that only a qualified individual, or a group, will be making the change.
If you have a strong case, the author will either make the changes for you, or will allow you to make changes to source text within specific parameters and/or with the author’s guidance.
Depending on the nature of the change, you may also need to adjust the scoring algorithm.
If the author, or the author’s representative or agent, refuse(s) to allow you to change source text even when you provide a solid argument for the change, your project or study may be better off with another instrument in its assessment schedule lineup.
Linguistic validation consists of translation and cognitive debriefing. If cognitive debriefing doesn’t take place, then it’s just a translation that is not linguistically validated.
The cost is built on the number of steps that are necessary to validate the translation. For a brand new translation, there are at least 2 full translations of the same document, plus at least 2 content reviews and editing.
Therefore, linguistically validated translations are more expensive than a classic translation from Language A to Language B.
Things to consider:
- Is linguistic validation necessary for the document you wish to have translated?
- Do you wish to start with an existing translation?
- Is the existing translation accompanied with a translation certificate that outlines its translation methodology?
- How long is the document?
- Are there any other documents that are dependent on the one that needs validation (e.g., administration and scoring instructions, visual aids, etc.)?
- Who is the target audience for cognitive debriefing?
- Have you allocated adequate time for cognitive debriefing?
The best way to get an accurate price for your translation project is to complete our Request for Quote form. Contact us and we will email it to you.
Once you submit it, we will provide you with a free quote with no obligations.
Clinical Outcome Assessments
the four Main types of Clinical Outcome Measures

PRO: Patient-Reported Outcome
Patient-Reported Outcome Measures capture patients’ direct reports of symptoms, functioning, and treatment experience without clinician or caregiver interpretation. In linguistic validation, we evaluate conceptual clarity, comprehension, response scales, recall periods, and item functionality. This helps support meaningful interpretation of data sets collected across international research sites.
ClinRO: Clinician-Reported Outcome
Clinician-Reported Outcome Measures are completed by trained healthcare professionals based on clinical judgment, direct observation, or structured rating procedures. Linguistic validation focuses on the clarity and precision of rating instructions, anchors, diagnostic terms, and scoring rules so clinicians interpret and apply each item consistently across countries. We also assess terminology, task descriptions, and administration guidance for the target population and local clinical context.
ObsRO: Observer-Reported Outcome
Observer-Reported Outcome Measures capture information from caregivers or observers about behaviors and symptoms that patients cannot reliably self-report. Linguistic validation focuses on the clarity of observable concepts, reporting instructions, and caregiver decision-making, ensuring that items are interpreted as intended across languages and cultural contexts. We also evaluate administration burden, examples, and task demands to prevent misclassification or inconsistent observation in multilingual studies.

PerfO: Performance Outcome
Performance Outcome Measures assess a patient’s ability to complete standardized tasks that measure specific aspects of functioning, such as motor skills, cognitive processes, or physical capability. In linguistic validation, we review the clarity of task instructions, stimuli descriptions, timing requirements, and scoring methods to avoid unintentionally altering task difficulty or administration. We also identify phrasing and cultural factors that unfairly impact participants' understanding and performance.
